All people today are classified as Homo sapiens. Our species of humans first began to evolve nearly 200,000 years ago in association with technologies not unlike those of the early Neandertals. It is now clear that early Homo sapiens, or modern humans, did not come after the Neandertals but were their contemporaries. However, it is likely that both modern humans and Neandertals descended from Homo heidelbergensis.
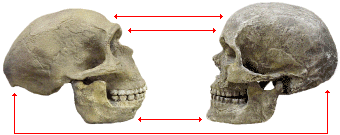
Compared to the Neandertals and other late archaic humans, modern humans generally have more delicate skeletons. Their skulls are more rounded and their brow ridges generally protrude much less. They rarely have the occipital buns found on the back of Neandertal skulls. They also have relatively high foreheads, smaller faces, and pointed chins.
|
|
|
| Neandertal | modern Homo sapiens |
Origins of Modern Humans
It would seem from these dates that the location of initial modern Homo sapiens evolution and the direction of their dispersion from that area is obvious. That is not the case. Since the early 1980's, there have been two leading contradictory models that attempt to explain modern human evolution--the replacement model and the regional continuity model.
The replacement model of Christopher Stringer and Peter Andrews proposes that modern humans evolved from archaic humans 200,000-150,000 years ago only in Africa and then some of them migrated into the rest of the Old World replacing all of the Neandertals and other late archaic humans beginning around 60,000-40,000 years ago or somewhat earlier. If this interpretation of the fossil record is correct, all people today share a relatively modern African ancestry. All other lines of humans that had descended from Homo erectus presumably became extinct. From this view, the regional anatomical differences that we now see among humans are recent developments--evolving mostly in the last 40,000 years. This hypothesis is also referred to as the "out of Africa", "Noah's ark", and "African replacement" model.
The regional continuity model (or multiregional evolution model) advocated by Milford Wolpoff proposes that modern humans evolved more or less simultaneously in all major regions of the Old World from local archaic humans. For example, modern Chinese are seen as having evolved from Chinese archaic humans and ultimately from Chinese Homo erectus. This would mean that the Chinese and some other peoples in the Old World have great antiquity in place. Supporters of this model believe that the ultimate common ancestor of all modern people was an early Homo erectus in Africa who lived at least 1.8 million years ago. It is further suggested that since then there was sufficient gene flow between Europe, Africa, and Asia to prevent long-term reproductive isolation and the subsequent evolution of distinct regional species. It is argued that intermittent contact between people of these distant areas would have kept the human line a single species at any one time. However, regional varieties, or subspecies, of humans are expected to have existed.
There are two sources of evidence supporting the replacement model--the fossil record and DNA. So far, the earliest finds of modern Homo sapiens skeletons come from Africa. They date to nearly 200,000 years ago on that continent. They appear in Southwest Asia around 100,000 years ago and elsewhere in the Old World by 60,000-40,000 years ago. Unless modern human remains dating to 200,000 years ago or earlier are found in Europe or East Asia, it would seem that the replacement model better explains the fossil data for those regions. However, the DNA data supporting a replacement are more problematical.
Beginning in the 1980's, Rebecca Cann, at the University of California, argued that the geographic region in which modern people have lived the longest should have the greatest amount of genetic diversity today. Through comparisons of mitochondrial DNA sequences from living people throughout the world, she concluded that Africa has the greatest genetic diversity and, therefore, must be the homeland of all modern humans. Assuming a specific, constant rate of mutation, she further concluded that the common ancestor of modern people was a woman living about 200,000 years ago in Africa. This supposed predecessor was dubbed "mitochondrial Eve"
Critics of the genetic argument for the replacement model also point out that the rate of mutation used for the "molecular clock" is not necessarily constant, which makes the 200,000 year date for "mitochondrial Eve" unreliable. The rate of inheritable mutations for a species or a population can vary due to a number of factors including generation time, the efficiency of DNA repair within cells, ambient temperature, and varying amounts of natural environmental mutagens. In addition, some kinds of DNA molecules are known to be more subject to mutation than others, resulting in faster mutation rates. This seems to be the case with the Y chromosome in human males.
Further criticism of the genetic argument for the replacement model has come from geneticists at Oxford University. They found that the human betaglobin gene is widely distributed in Asia but not in Africa. Since this gene is thought to have originated more than 200,000 years ago, it undercuts the claim that an African population of modern Homo sapiens replaced East Asian archaic humans less than 60,000 years ago.
Regional Continuity Model Arguments
Fossil evidence also
is used to support the regional continuity model. Its advocates claim
that there has been a continuity of some anatomical traits from archaic
humans to modern humans in Europe and Asia. In other words, the
Asian and European physical characteristics have antiquity in these regions
going back over 100,000 years. They point to the fact that many
Europeans have relatively heavy brow ridges and a high
angle of their noses reminiscent of Neandertals.
Similarly, it is claimed that some Chinese facial characteristics can be seen in
an
Asian archaic human fossil from
Jinniushan
dating to 200,000 years ago. Like Homo
erectus, East Asians today commonly have
shovel-shaped incisors while
Africans and Europeans rarely do. This supports the contention of direct
genetic links between Asian Homo erectus and modern Asians. Alan
Thorne of the Australian National University believes that Australian
aborigines
 share key skeletal and dental traits with pre-modern people who inhabited
Indonesia at least 100,000 years ago. The
implication is that there was no replacement by modern humans from Africa
60,000-40,000 years ago. However, the evidence does not rule out
gene flow from African populations to Europe and Asia at that time and before.
David Frayer, of the University of Kansas, believes that a number of European
fossils from the last 50,000 years have characteristics that are the result of
archaic and modern humans interbreeding.
share key skeletal and dental traits with pre-modern people who inhabited
Indonesia at least 100,000 years ago. The
implication is that there was no replacement by modern humans from Africa
60,000-40,000 years ago. However, the evidence does not rule out
gene flow from African populations to Europe and Asia at that time and before.
David Frayer, of the University of Kansas, believes that a number of European
fossils from the last 50,000 years have characteristics that are the result of
archaic and modern humans interbreeding.
Assimilation Model
Homo sapiens began migrating into the lower latitudes of East Asia by at least 70,000 years ago. Along the way, some of them interbred with archaic humans, including both Neandertals and Denisovans. Genetic markers from these archaic human populations are found in the gene pool of some Southern Chinese, New Guinean, and other Micronesian Island populations today. Homo sapiens from Southeast Asia travelled to Australia by 46,000 years ago and possibly as early as 60,000 years ago. Because Australia was not connected to Southeast Asia by land, it is probable that these first Australian Aborigines arrived by simple boats or rafts. Modern humans reached the Japanese Islands by 30,000 years ago or somewhat earlier. Around 35,000-30,000 years ago, Homo sapiens big game hunters moved into Northeastern Siberia. Some of them migrated into North America via the Bering Plain, or Beringia
A consequence of human migrations into new regions of the world has been the extinction of many animal species indigenous to those areas. By 11,000 years ago, human hunters in the New World apparently had played a part in the extermination of 135 species of mammals, including 3/4 of the larger ones (mammoths, mastodons, giant sloths, etc.). Most of these extinctions apparently occurred within a few hundred years. It is likely that the rapidly changing climate at the end of the last ice age was a contributing factor. However, the addition of human hunters with spears to the existing top predators (mostly saber-toothed cats, lions, and dire-wolves) very likely disrupted the equilibrium between large herbivores and their predators. As a consequence there was a major ecosystem disruption resulting in the rapid decline of both non-human carnivores and their prey. Humans were very likely the trigger that set off this "trophic cascade". Unlike most other major predators, people survived by switching their food quest to smaller animals and plants.
Following the arrival of aboriginal people in Australia and Polynesians in New Zealand there were similar dramatic animal extinctions. In both of these cases humans apparently were directly responsible for wiping out easily hunted species. Large vulnerable marsupials were the main victims in Australia. Within 5,000 years following the arrival of humans, approximately 90% of the marsupial species larger than a domesticated cat had become extinct there. In New Zealand, it was mostly large flightless birds that were driven to extinction by human hunters following their arrival in the 10th-13th centuries A.D.
It is sobering to realize that the rate of animal and plant extinction has once again accelerated dramatically. During the last century and a half, the explosion in our global human population and our rapid technological development has allowed us to move into and over-exploit most areas of our planet including the oceans. That exploitation has usually involved cutting down forests, changing the courses of rivers, pushing wild animals and plants out of farm and urban areas, polluting wetlands with pesticides and other man-made chemicals, and industrial-scale hunting of large land animals, whales, and fish. During the early 19th century, there were at least 40,000,000 bison roaming the Great Plains of North America. By the end of that century, there were only a few hundred remaining. They had been hunted to near extinction with guns. The same fate came to the African elephant and rhinoceros during the 20th century. Likewise, commercial fishermen have depleted one species of fish after another during the last half century. Governments have had to step in to try to stem the tide of these human population effects on other species. However, they have been only marginally successful. The World Conservation Union conservatively estimates that 7,266 animal species and 8,323 plant and lichen species are now at risk of extinction primarily due to human caused habitat degradation. The endangered list includes 1/3 of all amphibian species, nearly 1/2 of the turtles and tortoises, 1/4 of the mammals, 1/5 of the sharks and rays, and 1/8 of the birds. This list does not include the many millions of species that are still unknown to science. It is likely that most of them will become extinct before they can be described and studied.
People Today
It is not clear what all of the consequences of the environmental and behavioral changes for humans have been. However, it does appear that the average human body size has become somewhat shorter over the last 10,000 years, and we have acquired widespread immunity to the more severe effects of some diseases such as measles and influenza.
Finally, can we say what direction human evolution will take in the future? This is a fascinating question to consider but impossible to answer because of innumerable unknown factors. Though, it is certain that we will continue to evolve until we reach the point of extinction.